New gene therapy trial in Dayton offers hope for children with Canavan
Parents’ tenacity moves mountains to improve children’s quality of life
By Marshall Weiss, The Dayton Jewish Observer
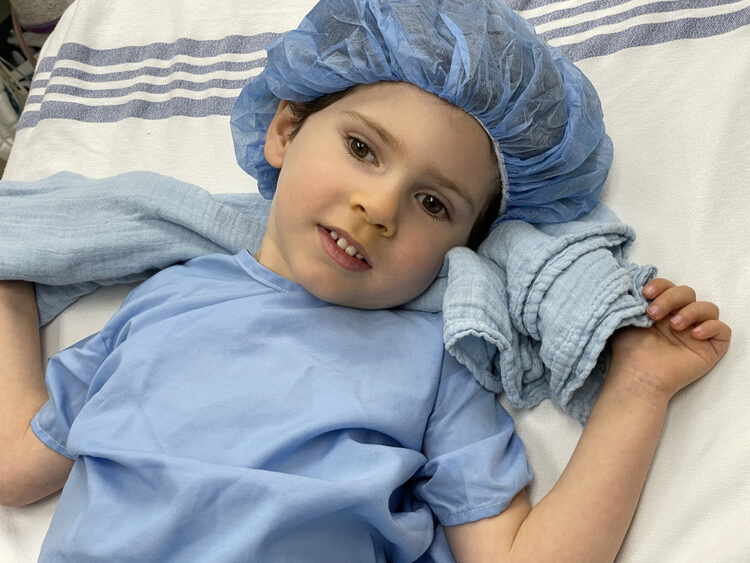
In an operating room at Dayton Children’s Hospital on April 8, 4-year-old Benny Landsman of Brooklyn, N.Y. received the first new clinical trial of an FDA-approved gene therapy for Canavan disease.
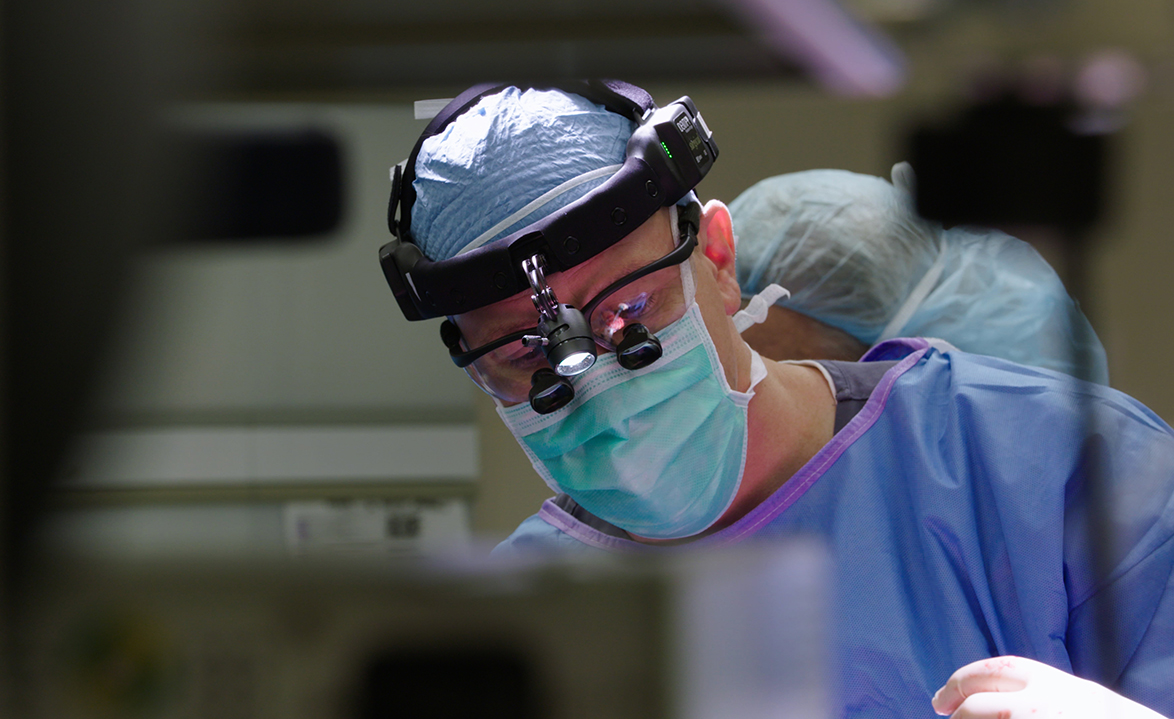
Neurosurgeon Dr. Robert Lober placed four catheters in holes drilled into Benny’s skull, down a track to a fluid space in the brain’s ventricles. Lober then manually injected a fluid containing 370 billion viral genomes of the new therapy.
By his side in the operating room were the trial’s clinical leader, Dr. Christopher G. Janson, also with Dayton Children’s and Premier Health’s Clinical Neuroscience Institute, and longtime Canavan researcher Paola Leone, professor of cell biology at Rowan University in Glassboro, N.J. and director of Rowan’s Cell and Gene Therapy Center.
Leone and Janson have worked for more than two decades to make this latest trial a possibility. But it is the hope and tenacity of Benny’s parents — and a generation of parents of children with Canavan — that made it a reality.
A year after Benny was born, Jennie and Gary Landsman had another son, Josh, who turns 4 in July. Benny and Josh were both diagnosed with Canavan when Josh was two weeks old.
Canavan is a debilitating, fatal neurological disorder in which the brain deteriorates because of a defective inherited gene. This mutated gene corrupts the body’s ability to produce myelin, the protective white matter in the brain.
Children born with Canavan rarely if ever survive beyond their first decade. They become blind, paralyzed, and prone to seizures. There is no cure.
A rare disease, Canavan is more frequently found in people of Ashkenazi (Eastern European) Jewish descent. One in 40 Ashkenazi Jews is a carrier for Canavan.
Neither Benny nor Josh can walk or talk. Josh can still roll over. Benny is on a feeding tube.

“They’re sweet kids and they smile, and they have personalities. They’re definitely aware,” Gary said. “But they have virtually no control of their limbs, they have no speech, they can’t sit up or even hold their heads up for more than a few seconds, if that.”
Benny and Josh are able to communicate through an eye-tracking device; they look at images on a computer display to let their parents know what they need.
Jennie Landsman had gone through genetic testing before she gave birth to Michael, her son from her first marriage. Her doctor told her she carried no markers for genetic diseases and had nothing to worry about. With Michael, she didn’t. But with her second and third sons, the Landsmans would find out either the test or the doctor was wrong.
“When we thought that Jennie didn’t have any markers, we didn’t think it was important,” Gary said of the couple’s thoughts on genetic testing. “We didn’t think she had to go through additional testing, we didn’t think I had to go through any testing.”
At the Landsmans’ first meeting with a geneticist to discuss Benny and Josh’s diagnosis, they were told nothing could be done for the boys, to make them as comfortable as possible until they passed.
“It probably took us about a month or two before we crawled out of the deep, dark hole that we were in when we found out the news,” Gary said.
Jennie read everything there was to read about Canavan, connected with other Canavan families online, and reached out to Leone in fall 2017.
Leone had conducted two previous clinical trials for Canavan, in 1998 and 2001. Janson collaborated on the 2001 trial. Those trials targeted neurons. Although they saw some improvements in patients, such as fewer seizures, Leone and Janson discovered they were targeting the wrong cells.
By 2017, Leone and Janson had developed a new gene therapy using a “modified recombinant Adeno-Associated – Viral Vector.” The lab-produced gene targets the cells that are significant for white-matter development.
To bring the gene therapy from the lab to clinical trials, Leone estimated the project would cost $1 million.
That Thanksgiving, the Landsmans opened a GoFundMe page. In a matter of weeks, they raised $1.5 million.

At first, Leone and the Landsmans expected the boys would receive the new clinical trial in April 2018. Updated FDA requirements ultimately raised the cost to more than $5 million and, combined with the Covid pandemic, would delay the procedure another three years.
With expertise from a mom who had already been there, the Landsmans managed to achieve total funding with donations from more than 18,000 donors: not only for Benny and Josh, but for the 24 estimated participants needed to complete the first trial.
“Perhaps it was good that we were told six, seven months and a million dollars and I’ll treat your kids,” Gary said. “Had we been told from day one that we would need to raise $5 million, that it’s going to take over three years to get there, those numbers and that time frame may have been a little too daunting to us.”
Janson said he and Leone did their best to estimate the trial’s cost. “But I guess we were a bit naive about all the changes in the regulations and requirements now,” Janson said. “There’s a lot of red tape that wasn’t there before.”
One factor in 2018 was a change because of a contamination in genetic trial materials unrelated to their proposed clinical trial.
Leone submitted two separate grants to the National Institutes of Health twice between 2018 and 2020 but didn’t receive sufficient support from members of the NIH Study Section to receive funding.
“The grant application received a low impact score mostly on significance,” said Leone, who holds a doctorate degree in neuroscience from the University of Padua.
Janson said NIH’s rejection was likely in part because with so few cases of Canavan, few individual lives would be directly improved from the gene therapy.
“Also, a lot of the NIH funding process is about interesting science rather than practical applications,” he added. “It’s ironic but it’s kind of the reality. And the FDA is not really supporting compassionate trials anymore.”
Lober said that although the research’s focus is on a very rare disease, this type of gene therapy could be applied to numerous white-matter diseases.
“There are lots of other diseases that we could potentially have applications for based on what we’ve learned from this,” Lober said. “And so therefore, it’s not just hope for these folks, but for so many people. That’s what I think is amazing about this.”
If Leone and Janson had waited for the next NIH grant cycle, they wouldn’t be able to treat patients until 2023, following the 12-month timeline for drug manufacturing.
Benny, who turns 5 at the end of June, would have been too old for this clinical trial after his coming birthday.
“The way the trial is designed, there are very specific age groups we can work with,” Lober said. “The maximum age is 5.”
If not for the Landsmans, the clinical trial would still be waiting for funding.
Eighteen patients are on the waiting list for the clinical trial and 10 doses have been manufactured, with another batch on the way.
“We could not have done it without them,” Leone said of the Landsmans. “If everything goes well, we’re going to have one patient every month after we clear the first safety group — which is made of three patients — until we reach February 2022 and then start with a new batch of vector if we have sufficient time to produce it.”
Benny’s little brother, Josh, is on the list to receive the second clinical trial, at the beginning of June. Gary said it isn’t official yet, but right now it looks like it’s going to happen.
Leone said the therapy’s success will be based on its ability to improve motor function in these children.
“We are hoping to complete the trial with benefits, with safety and efficacy on the study in about three years,” she said.
The clinical trial continues to recruit children with Canavan ages 3 months to 60 months.
All clinical trials for phases one and two will take place at Dayton Children’s. Janson moved to Dayton a year and a half ago from Chicago to join the Clinical Neuroscience Institute, a Premier Physician Network practice.
“The hospital where it was going to happen was not ideal for a lot of reasons,” Janson said. Lober came to Dayton Children’s six years ago. “Rob came into the picture and he’s fantastic,” Janson added. “We didn’t have a pediatric neurosurgeon and now we’ve got a Stanford-trained pediatric neurosurgeon to make this happen. So I think it was fortuitous and came together.”
“I have to say that I’ve been to Yale (New Haven Hospital), I’ve been to CHOP PICU (Children’s Hospital of Philadelphia’s pediatric intensive care unit), and Jefferson (University Hospital), and many other hospitals, and I’ve never seen anyone who does it like Dr. Lober and his team, managing before, during, and after,” Leone said.
If the trial is beneficial to its patients and it’s sufficiently safe through its third and final phase, Leone said she’ll develop it into an approved product for Canavan disease.
“If the product goes to phase three, we could identify other sites and then once it’s approved by the FDA and then by the EU, it could be used by any well-trained surgeon all over the world,” Leone said.
Jennie and Gary attribute their success in actualizing the clinical trial to divine intervention.
“It kind of snowballed and we really did not have any plan or special abilities,” Jennie said.
After their GoFundMe video went viral and with the first $1.5 million coming in, Jennie received a call from Jordana Holovach.
Two years earlier, Holovach’s 19-year-old son, Jacob, died of Canavan. She had raised money for Leone’s two earlier gene therapy trials, founded a Canavan charity, and consults for rare-disease funding.
“She called me out of the blue and just said, ‘I want you to know you’re doing a great job and I’m here for you if you ever need anything,’ Jennie said of Holovach. “And then one day, I needed her.”
Holovach helped the Landsmans establish the Cure Canavan Fund 501(c)(3) to get them the rest of the way and to help other families raise money for the clinical trials. She now serves as the clinical trial’s patient advocate.
She leveraged the Landsmans’ initial success with GoFundMe into more video content exposure on social media with a targeted focus on Jewish communities around the world.
“Little by little, with the Landsmans and other families from Russia, Slovakia, Italy, Poland, we were able to accomplish the task to complete the manufacturing with multiple doses and to have parents start their clinical trials,” Leone said.
“I remember being that parent: getting the worst diagnosis of your life, being told there is nothing else out there, there’s nothing you can do,” Holovach said. “I know this journey. Mostly I’m contacted by rare-disease groups because that’s what I lived through with my son. I don’t want to be an expert in this, I want cures.”

When the team hit roadblocks that delayed the FDA’s approval, Holovach secured a meeting at FDA headquarters for the Landsmans and their clinical team in April 2019. Jennie and Gary brought sons Benny and Josh with them.
“We brought the boys because we wanted them to see that there are two real children waiting, and that they’re not just a case number,” Jennie said.
“All projects need a project manager to push all the various players along and fortunately we were able to be in touch with them and get a project manager on their end, and both Jennie and I communicated with the FDA on a regular basis to really try to push things forward,” Gary said.
The Landsmans arrived at an Airbnb 20 minutes from Dayton Children’s for their stay during Benny’s April surgery and recovery. With them were Jennie and Gary’s other children, Michael (11), Evan (2), and Racheli (6 months), and two home healthcare aids to assist with Benny and Josh.
Early in Jennie’s pregnancies with Evan and Racheli, the Landsmans confirmed neither had Canavan.
Benny’s procedure was scheduled for the intermediate days of Passover. The Landsmans reached out to Chabad of Greater Dayton; its co-director, Devorah Leah Mangel, prepared all the dinners for the family over their two-and-a-half weeks in Dayton.
“Right away, they said, ‘We’re taking care of you. We don’t want you to have to think about a thing,’ and they made sure we had beautiful meals with lots of love and care put into every single one,” Jennie said of Dayton’s Chabad.
Along with visits from Jennie’s sister and parents (Gary’s parents live in Israel), the Landsmans’ rabbi, Etan Tokayer with Kingsway Jewish Center in Brooklyn, flew in for the day of the surgery.
The Landsmans returned to Dayton Children’s for a follow-up May 3. The couple said Benny has recovered much quicker than they had expected.
Since Benny is the first person to receive this trial, no one knows when to expect clinical benefits, if any.
“It might be three or six or 12 months until any clinical benefit,” Janson said. “We’re not exactly sure.”

“It could yield no results, it could potentially be harmful for our kids,” Gary said. “We recognized that, and that was one of the things we talked about with the FDA, that we’re going into this with our eyes wide open.
“But if they even have any chance of having an easier time communicating or having an easier time swallowing or have some mobility — if any of those are real possibilities — then we have to try whatever it is we can do. As God-fearing people and God-loving people, we believe that God is capable of anything. So we really have no idea what to expect, but we did know that we couldn’t sit still and do nothing.”
On this side of the procedure, the Landsmans have the gift of hope. And gratitude.
“We’ve been beyond moved by the generosity of people, and I’m not speaking exclusively to the financial support that we’ve gotten from people, but the chesed,” Gary said, using the Hebrew term for acts of lovingkindness. “We’re not completely sure what our lives’ mission is going to be. But we do know that we have to pay it forward. We absolutely have to. We want to, and it’s changed us.”
To read the complete June 2021 Dayton Jewish Observer, click here.

